Systematic reviews and meta-analyses help to save time in research and provide a resourceefficient methodology that leads to an improvement in the quality of science and animal welfare and thus to an increased implementation of the 3R.
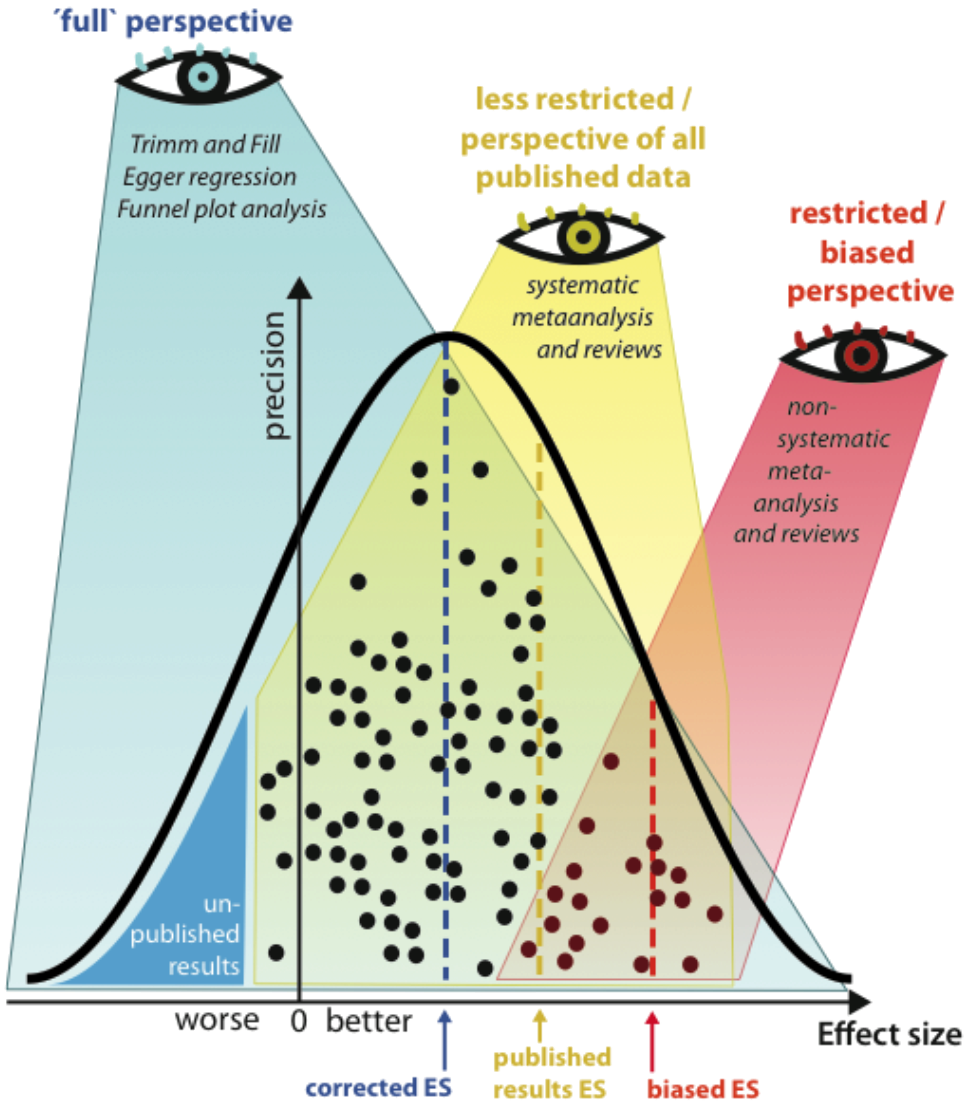
The chances of success (correct prediction) of translation can be improved by a strategic, systematic and transparent approach: with the help of an evidence-based methodology (e.g. meta-regression, Egger regression, trim-and-fill algorithm), systematic reviews and metaanalyses go beyond the techniques of classical narrative systematic reviews. This can significantly address the problem of "publication bias" (Figure 1). Applying an advanced metaanalytic methodology to animal studies to identify, evaluate, select and synthesise all available high-quality research evidence offers the possibility to determine concrete effect sizes for specific therapeutic approaches and thereby critically assess the need for further animal experiments.
If experiments (dots) have been conducted but are not avallable to reviewers, and in case the results of these experiments as a group are different from the results of published experiments, then both, non-systematic (red eye perspective) and systematic reviews yellow eye perspective), as well as expert opinions and public understanding will be biased (modified from 12). The resulting effect sizes yellow and red line) suggest large effect Sizes, which are 'inflated' and represent overestimations. Together, here we confine 'non-systematic',
'svstematic' and 'trim and fill analvsis to a hierarchy of 'filedrawer
perspectives, from a restricted, less restricted to a non restricted 'full perspective. A strategy to visualize missing data (e.g. due to a negative publication bias; indicated by blue triangle on the left ('unpublished results) is to apply the funnel plotting method and Egger regression, and the 'Duval and Tweedie non-parametric trim and fill' approach 16, 17 (blue eye perspective).
In recent years, the following main scientific findings have been made:
The effect sizes reported in individual animal studies were usually about 30% higher than can be demonstrated in systematic reviews and meta-analyses. The main reasons for this were limited study quality, such as lack of randomisation or blinding.
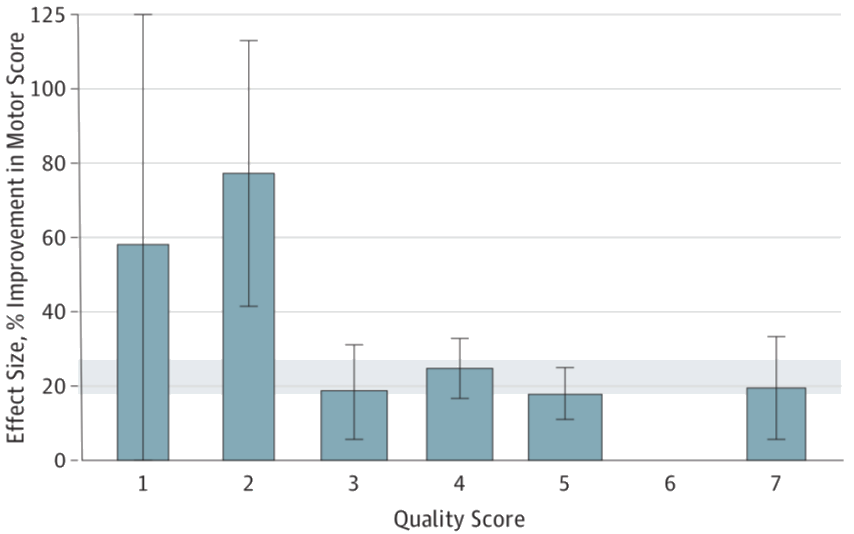
The individual study quality was determined by means of a 9-point quality score. In addition to methodological aspects (publication in peer-reviewed journals, compliance with animal welfare standards, etc.), animal experimental aspects (blinding, randomisation, etc.) are included in the score. We were able to show that a high (good) study quality is associated with a reduced effect size.
Systematic reviews and meta-analyses represent an essential contribution to current research in the assessment of therapy effects. In particular, the investigation of "publication bias", which systematically distorts individual study results, is of great interest here. Modern statistical methods can be used to detect this in the field of experimental research.
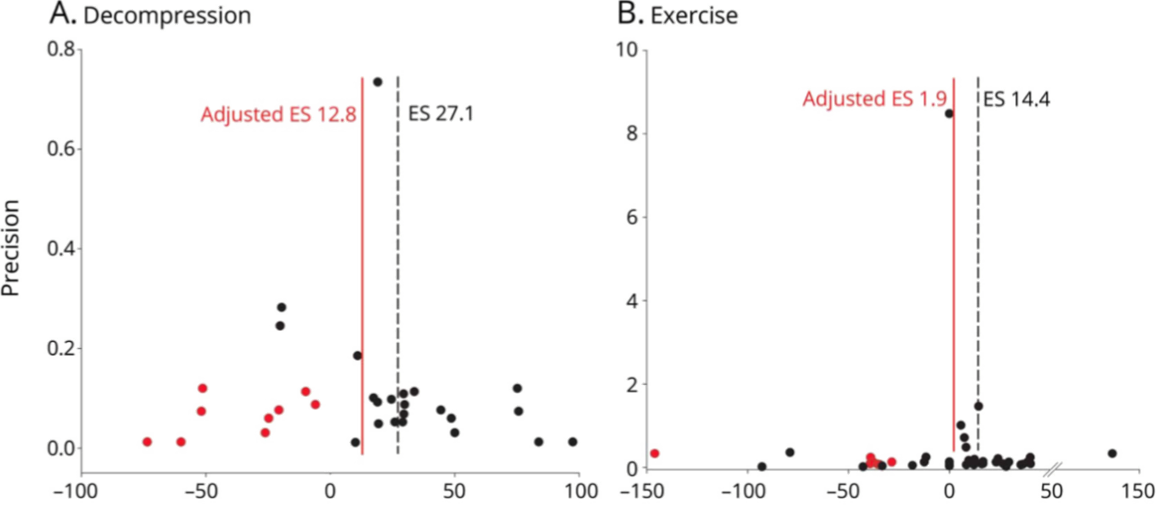
In a large case analysis with a total of 9535 animals with SCI
Background SCI:
In the field of traumatic spinal cord injury (SCI), no effective treatment option for humans has been demonstrated to date. Approximately 580,000 new patients are diagnosed with SCI each year worldwide, and an estimated 1.5-5.2 million patients suffer sequelae after SCI. Despite increasing optimism, largely justified by increased molecular and cellular knowledge, no effective intervention has yet been found in the setting of human SCI. The lack of therapeutic options for these patients points to the need to develop validated therapeutic interventions after SCI and encourages translational efforts. Compared with other central nervous system (CNS) disorders, clinical translation in SCI leading to interventional trials is relatively new and depends significantly on projects that contribute to an ongoing bidirectional "translational dialogue. "9-11 However, translating evidence from preclinical work to humans is a major challenge and recent scientific publications indicate that the translational value of animal models for clinical trials should be evaluated. , we were able to demonstrate "publication bias", which leads to an estimated between 2 and 41% of preclinical experiments remaining unpublished. These experiments are performed in the laboratory, but are presumably mostly not reported due to negative results (publication bias). )
The individual effect sizes are plotted in funnel plots. In a case series with 9535 animals, we were able to demonstrate publication bias, which leads to an "overestimation" of the overall effect size. After adjusting for publication bias, the effect sizes were reduced in both examples shown, in example A by 14.3% and in example B) by 12.5%.
An example of the failure of clinical translation could be seen in two large, well-designed clinical trials on the efficacy of progesterone in severe traumatic brain injury. severe traumatic brain injury (number of patients recruited in both studies: 2077 patients). both studies: 2077 patients). Six years before the publication of the patient studies a systematic review & meta-analysis of the preclinical efficacy of progesterone in animal models was published. Based on these data, we were able to methodological weaknesses that should have been taken into account in the design of the clinical trials. should have been taken into account. This is a possible reason for the failure of the two large studies and reinforces the importance of the preclinical meta-analyses.